Overview
Hepatic encephalopathy (HE) is a frequent complication of cirrhosis. It is defined as brain dysfunction caused by liver insufficiency and/or portosystemic shunting that manifests as a wide spectrum of neurological or psychiatric abnormalities, ranging from subclinical alterations to coma. HE is classified as covert or overt based on severity of symptoms along this spectrum.1
HE ranges in severity from minimal to overt1

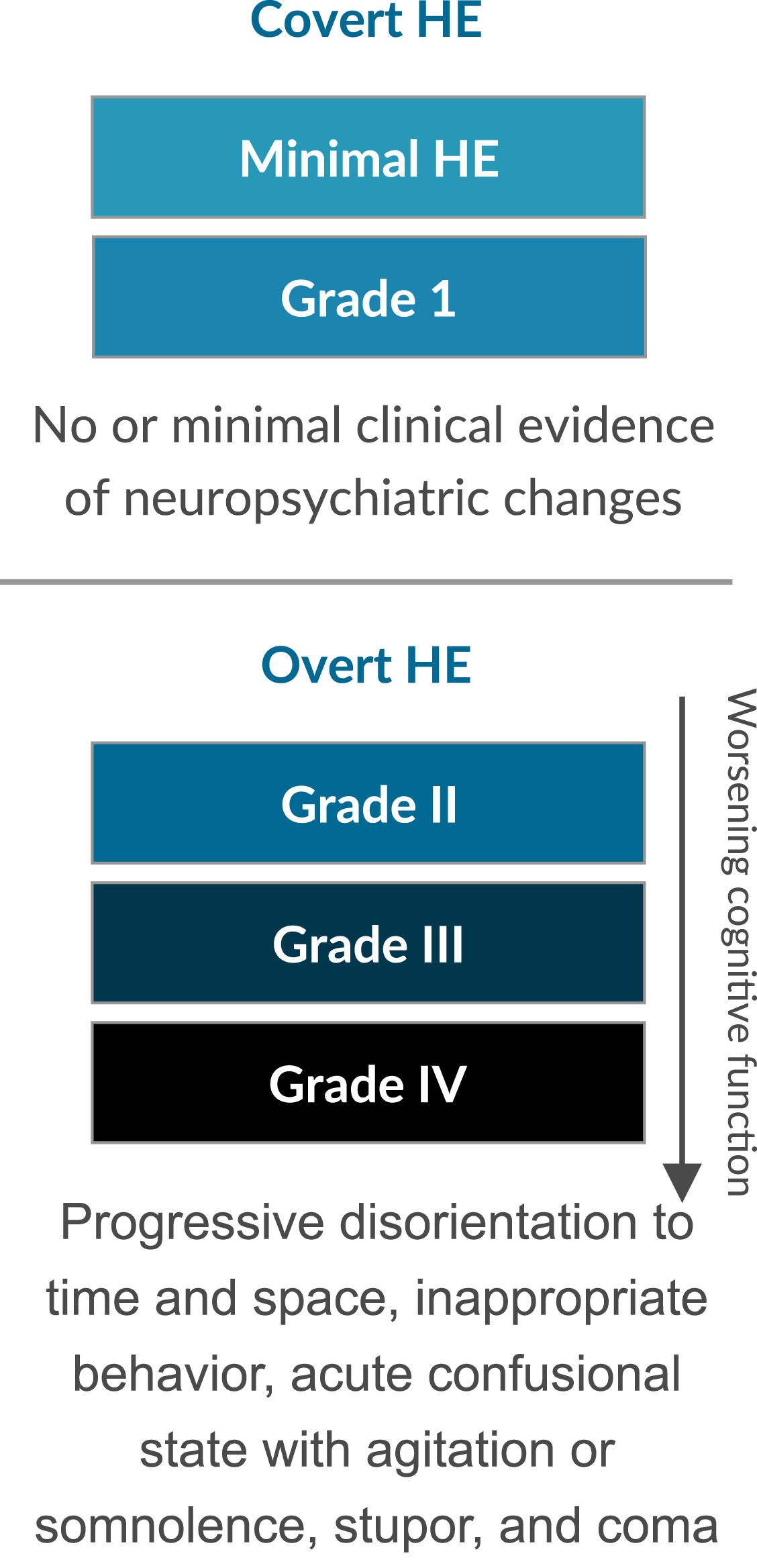
HE that is fully symptomatic is classified as overt HE, which represents the decompensated phase of cirrhosis and is typically recurrent or persistent.1


Medical Unmet Need in Overt HE


- *Retrospective claims data from a regional Medicare Advantage plan in Texas. Patients included those with hospital admissions or emergency department visits for HE between 2011 and 2018 who had either a medication refill for HE or an outpatient visit ≤30 days after hospital discharge (n=184).
- †Medications included lactulose, rifaximin, and neomycin.
- ‡Adherence varied dependent on medication used and was based on percentage of days covered by medication.
- §Hospitalization rate at 90 days was 14.4% (n=15/104) in patients taking medication and 20.0% (n=12/60) in those not taking medication.
- Source: Vadhariya A, et al. Medicine. 2020;99(16):e19603.
Pathophysiology of HE
Prolonged cycles of injury and repair progressively scar the liver and limit liver function,2 affecting both the liver tissue and underlying vasculature.3 Portosystemic shunting is a common consequence of prolonged portal vein hypertension, causing the diversion of unfiltered blood flow away from the liver to the systemic circulation.3 Cirrhosis also greatly impairs the ability of the liver to adequately break down toxins, causing the accumulation of neurotoxic and inflammatory agents.3


Reduced toxin clearance associated with liver dysfunction has been commonly studied as a pathological cause of HE. For decades, investigations focused on ammonia as the primary toxin implicated in HE; however, recent evidence suggests additional toxins as potential contributing factors. Elevated levels of endogenous benzodiazepines, possibly associated with gut bacteria, have been found in some patients with cirrhosis and HE. Although endogenous benzodiazepines can activate GABA-A receptors and are associated with astrocyte swelling, their role remains uncertain. The composition of fecal bacteria has been associated with HE pathogenesis, with a reduced level of bacteria associated with short-chain fatty acid production.4 Lipopolysaccharide (LPS) is an endotoxin derived from intestinal bacteria. Research identified endotoxemia (circulating LPS) as a predictor of liver disease, and higher levels of endotoxemia have been found in HE patients.5
Persistent or repeated episodes of HE can lead to profound, potentially irreversible, brain damage over time that manifests as the array of psychoneurological symptoms prevalent in overt HE.6
VIEW CONGRESS MATERIALS AND JOURNAL ARTICLES ON OVERT HE.
Learn more about irritable bowel syndrome (IBS) with diarrhea (IBS-D), IBS with constipation (IBS-C), and chronic idiopathic constipation.
References
-
Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715-735.
-
Khan A, Ayub M, Khan WM. Hyperammonemia is associated with increasing severity of both liver cirrhosis and hepatic encephalopathy. Int J Hepatol. 2016;2016:6741754.
-
Philips CA, Rajesh S, Augustine P, Padsalgi G, Ahamed R. Portosystemic shunts and refractory hepatic encephalopathy: patient selection and current options. Hepat Med. 2019;11:23-34.
-
Bloom PP, Luévano JM Jr, Miller KJ, Chung RT. Deep stool microbiome analysis in cirrhosis reveals an association between short-chain fatty acids and hepatic encephalopathy. Ann Hepatol. 2021;25:100333.
-
Männistö V, Färkkilä M, Pussinen P, et al. Serum lipopolysaccharides predict advanced liver disease in the general population. JHEP Rep. 2019;1(5):345-352.
-
Limón ID, Angulo-Cruz I, Sánchez-Abdon L, Patricio-Martínez A. Disturbance of the glutamate-glutamine cycle, secondary to hepatic damage, compromises memory function. Front Neurosci. 2021;15:578922.


